GAS CHROMATOGRAPHY
GAS CHROMATOGRAPHY
- It is used for the separation as well as analysis of gaseous mixture and volatile organic compounds.
Principle
- The components of a mixture are separated depending upon the extent of adsorption or partition of the stationary phase and at the rate at which the component is carried out by the mobile phase.
- The mobile phase is a gas like Argon, Nitrogen, Helium, or hydrogen. The stationary phase may be solid or liquid.
- If the stationary phase consists of a solid material like silica, alumina, then chromatography is termed as Gas Solid Chromatography (GSC), and if the stationary phase is a liquid held as a thin layer on a solid support, then the technique is known as Gas-Liquid Chromatography (GLC)GSC- adsorption. GLC- partition
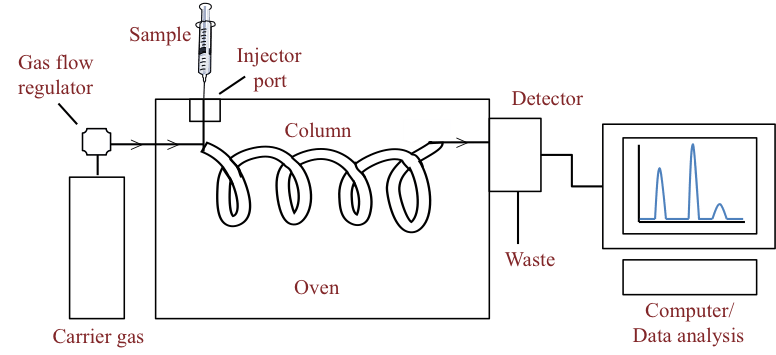
INSTRUMENTATION
- Carrier gas: It is allowed to flow through the system, carrying the sample in the vapor state through the column. It should be chemically inert, suitable for the detector employed, it should give the best column performance with the required speed of the analysis.
- Sample injection system: It is connected to the gas reservoir to the sample port injector. The sample must be converted to the vapor state. The injection port is heated to a temperature that will ensure rapid vaporization. The sample is injected by syringe. A solid sample may be dissolved in a suitable solvent and injected as a solution. The solute vapor mixes with the flowing carrier gas.
- Columns: The different components in the vaporized samples are separated from each other by their difference in interaction with the column packing. Columns are made of stainless steel, nickel, or glass.
- a) Capillary column: The inner surface is coated with a very thin film of the liquid phase. Ex- dimethylpolysiloxane and carbowax 20M are used. The characteristic feature of an ideal liquid stationary phase is low volatility, thermal stability, and chemical inertness
- b) The packed columns: They are packed with a solid powder substrate or liquid coating on an inert solid support. Materials used as solid stationary phase should have a high surface area and chemical inertness like finely powdered silica or alumina.
Detectors: Based on the various physical properties, detectors are of various types
a) Flame Ionization Detector (FID)
These are based on the electrical conductivity of gases. With a burner, the effluent from the column is mixed with the hydrogen gas and then ignited electrically. Most organic compounds when pyrolyzed produce ions and electrons that can conduct electricity through the flame.
b) Thermal conductivity detector (TCD)
The principle is based on the rate of heat loss from a heated wire placed in a gas stream which depends on the thermal conductivity of the gas, so the temperature of the wore changes, consequently the resistance. TCD responds to all types of organic and inorganic compounds those not detected by FIDIt does not destroy the eluted components. It is less sensitive than FID.
PROCEDURE
- The sample mixture is injected into the injection port where it gets vaporized and carried by the carrier gas into the column.
- During the process, the components of the mixture get distributed between two phases depending on the extent of partition or adsorption.
- In this way, different components are carried at different rates and they emerge from the column at different times.
- The time taken by each component to pass through the column is a characteristic property that helps for its identification.
- They are then detected by the detector, in which the recorder gives a peak for each component. The size and location of the peak is an indication of the nature of the peak.
Comments
Post a Comment