HIGH PERFORMANCE LIQUID CHROMATOGRAPHY
HIGH-PERFORMANCE LIQUID CHROMATOGRAPHY
It is a method used for the separation, purification, and identification of various nonvolatile high molecular weight organic compounds and natural products like cholesterol, terpenoids.
ADVANTAGES
- High-speed separation
- Excellent column separation
- Solvent consumption is minimum
- Sensitive and accurate
- Separation can be done at an ambient temperature.
PRINCIPLE
The components of the mixture are separated depending upon the extent of adsorption and partition on the stationary phase and the rate at which the component is carried by the mobile phase. The mobile phase is liquid and the stationary phase can be solid or liquid.
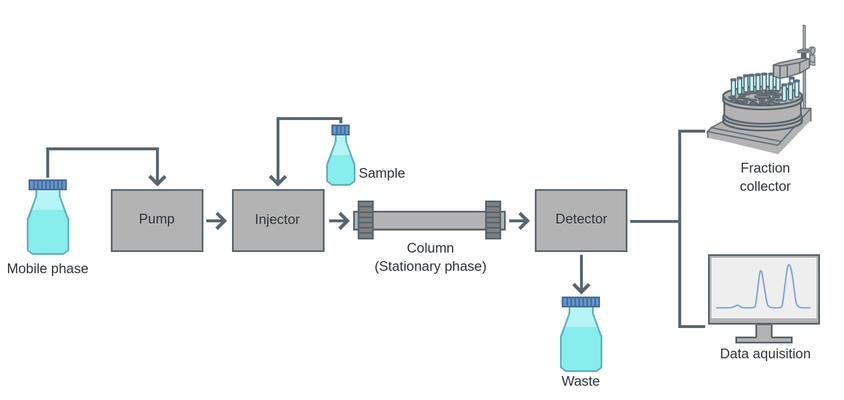
INSTRUMENTATION
- Solvent reservoirs are used to collect the different solvents.
- Pressure pump to apply pressure
- Sample injector to give the mixture which is to be separated in the analytical column
- Regulator to adjust the flow rate
- Guard column to remove the particulate matter and contaminants from the solvent.
- The analytical column is a long narrow, smooth stainless steel column with a small diameter. The column is packed with adsorbent material of reduced particle size.
- Detector
- Bulk property detector which corresponds to the bulk properties of the mobile phase like refractive index, density, dielectric constant etc. which is modulated by the presence of solutes. As the components pass through the column a respective signal will be produced.
- Solute property detector represents the properties of solutes such as UV absorbance, fluorescence which are not possessed by the solvent mobile phase. The solute property detectors give the spectra of the mixture.
PROCEDURE
The liquid mobile phase is pumped at the required pressure and flow rate through the analytical column which contains the stationary phase. The non-volatile samples are injected into the analytical column with the help of a microsyringe. The components are carried along the column at different rates due to the differential distribution between the stationary and mobile phases.
Elution can be done in two ways
- Isocratic elution is done by using a single solvent of constant composition.
- Gradient elution is done by using two or more solvents that differ in their polarities. They are kept in separate reservoirs and their mixing ratio can be suitably programmed.
APPLICATION
- Quality control
- Process control
- Forensic analysis
- Environmental monitoring
- Clinical testing
- Biomedical research work.
❤️❤️❤️
ReplyDelete