COLUMN CHROMATORGAPHY
COLUMN CHROMATOGRAPHY
- It is a technique in which the stationary phase is solid or a liquid supported by a solid.
- When a column is used as the adsorbent it is called adsorption column chromatography.
- When the solid column is acting only as a support to the liquid adsorbent then it is called partition column chromatography.
SEPARATION
The selection of solvent is based on the nature of the components in the mixture. Adsorption depends upon the nature of the solvent and the adsorbent.
The rate at which the components of a mixture are separated depends upon the activity of the adsorbent and the polarity of the solvent.
If the activity of the adsorbent is very high and polarity is low, then separation is low but gives good separation.
If the activity of the adsorbent is low and the polarity of the solvent is high, then the separation is rapid but gives poor separation
PROCEDURE
- A proper adsorbent is selected and made a slurry with a suitable liquid and placed in a suitable tube which is plugged at the bottom with glass wool.
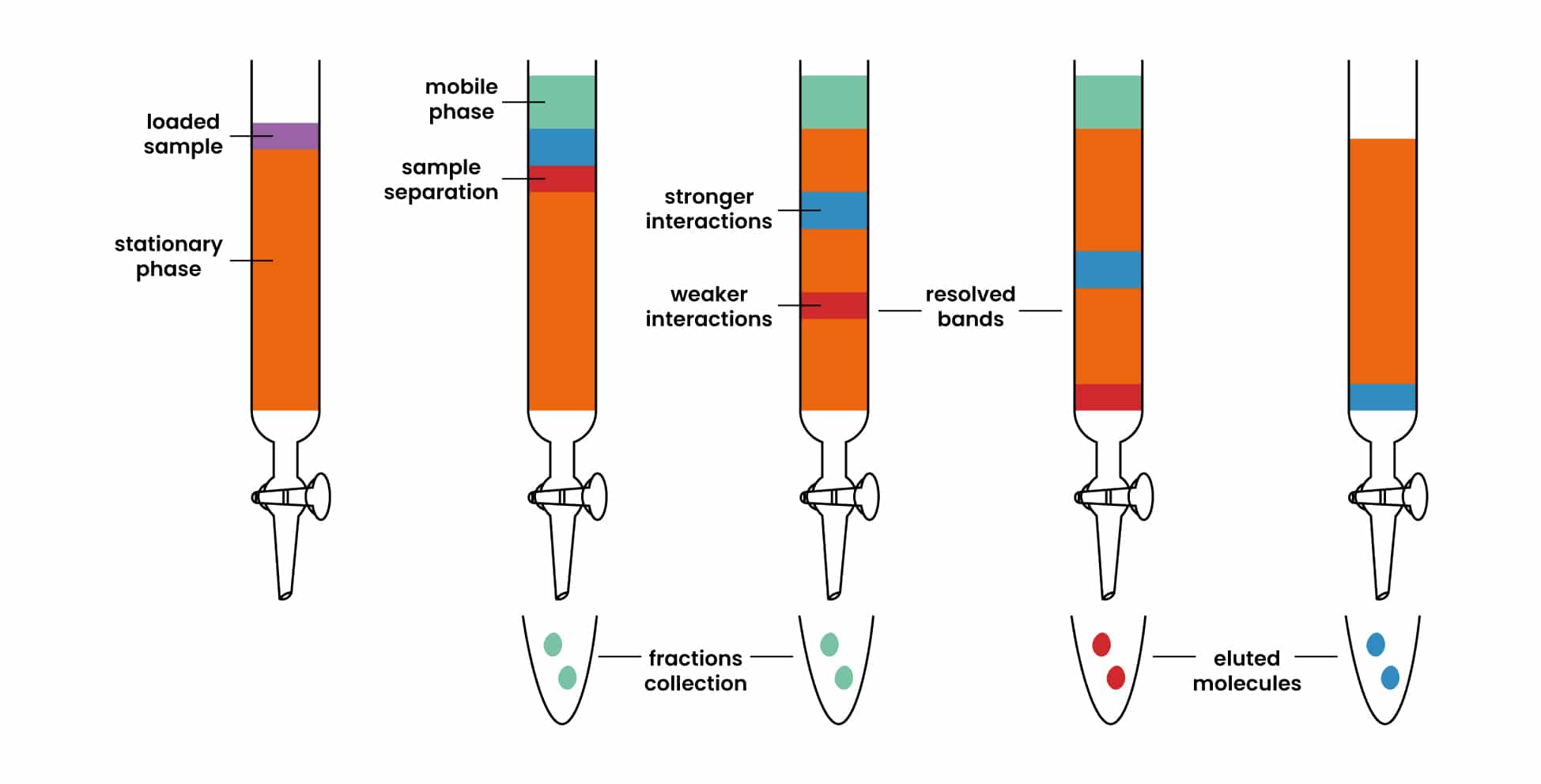
- The mixture which is to be separated is dissolved in a suitable solvent and introduced at the top of the column and is allowed to pass through the column, the components are adsorbed at different regions depending on their ability for adsorption.
- The one with greater adsorption power will be adsorbed at the top and the one with lower adsorption power will be adsorbed at the bottom.
- The different components can be collected separately by adding more solvent at the top and this process is known as elution.
- The weakly adsorbed component will be eluted more rapidly than the other. The different fractions are collected separately.
- The banded column of adsorbed constituents is called a chromatogram.
- The components which leave the column pass through a detector and recorder. The detector produces an electric signal and the recorder converts the signal to a trace on paper. The resulting plot of the signal intensity against time is called a chromatogram.
APPLICATION
- Quantitative separation of two or more components in a mixture.
- Purification of substances from their components.
- The concentration of solutes from their dilute solutions.
- Identification of technical products.
❤️
ReplyDelete