ELECTRONIC TRANSITION
ELECTRONIC TRANSITION
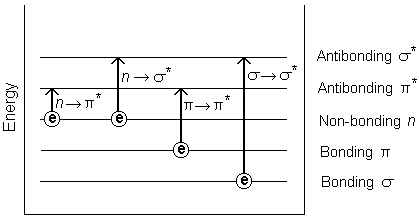
1. σ-------> σ* transitions
An electron is excited from the bonding σ orbital to the corresponding σ* orbital. The transition energy required is very high. This type of transition occurs below 150nm. Therefore the absorption occurs in the vacuum UV region. This type of transition occurs in saturated hydrocarbons. Thus saturated hydrocarbons do not show any absorption in the UV, visible region. Therefore they are colorless and known as UV transparent compounds.
2. π-------> π* transitions
The unsaturated hydrocarbons containing double or triple bond shows this type of transition.
The π ---->π* bands appear at 180-190nm in the case of aliphatic compounds and at 200-210nm in the case of simple aromatic compounds.
Eg: Ethene-190nm, 1,3-butadiene – 217nm, 1,3,5-hexatriene – 247nm, Benzene- 255nm
3. n-------> π* transitions
These transitions are observed in aldehydes and ketones which contain the C=O group which has both π electrons and non-bonding electrons (oxygen atom). It occurs in the range of 270-300nm with low intensity. (Symmetry forbidden)
4. n-------> σ* transitions
Compounds having non-bonding electrons like oxygen, sulfur, nitrogen, or halogen atoms show absorption in this transition. These transitions are also forbidden and occur in low intensity. The absorption takes place below 200nm
Comments
Post a Comment