BEER LAMBERTS LAW
BEER LAMBERTS LAW
When a beam of monochromatic EM radiation is passed through an Absorbing solution of concentration (c), the rate of decrease in intensity(-dI) of radiation with a thickness of the solution (dx) is proportional to the intensity of the radiation (I) and concentration (c) of the solution.
-dI/dx ∝ Ic
-dI/dx = kIc
-dI/I =kcdx
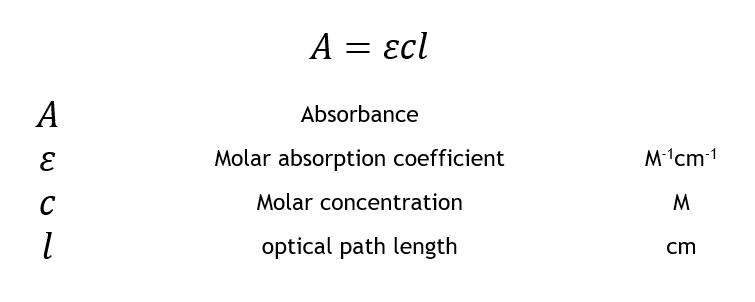
Beer Lambert's law gives the linear relationship between the absorbance of EM radiation and the concentration of an absorber.
A = εcL
A is the absorbance
c is the concentration
L is the path length
Ε is the molar extinction coefficient
Comments
Post a Comment