STANDARD HYDROGEN ELECTRODE
STANDARD HYDROGEN ELECTRODE
The standard hydrogen is an example of a glass electrode, used as a primary reference electrode whose electrode potential is fixed as zero.
CONSTRUCTION OF SHE
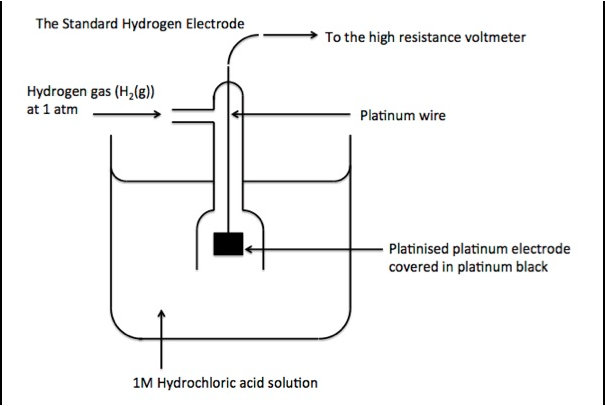
- Hydrogen gas at 1atm pressure is bubbled through a one molar H+ ions as HCl solution at a temperature of 298⁰K. Under these conditions both hydrogen as well as hydrogen ions will be in their standard state and hence the name standard hydrogen electrode.
- The platinum wire makes electrical contact and it is surrounded by an outer glass tube that has an inlet for hydrogen gas at the top and a number of holes at the base for the escape of hydrogen gas.
- It can act as a cathode as well as an anode depending on the potential of the electrode to which it is coupled.
- If the potential of the coupled electrode is less than zero (Zn, Mg, Li) reduction takes place in SHE and it acts as the cathode.
- If the potential of the coupled electrode is greater than zero (Cu, Ag) oxidation takes place in SHE and acts as an anode.
Comments
Post a Comment